Electroleaching and electrodeposition of silver in ethaline 1:2 and propeline 1:3
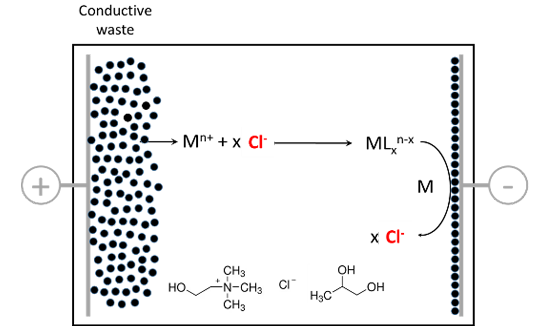
This data set is related to a published research article dealing with electrochemical leaching and electrochemical deposition of silver in two deep-eutectic solvents (DES) : ethaline 1:2 and propeline 1:3.
The objective was to compare the performances of the two DES, propeline 1:3 being much less toxic than ethaline 1:2. Ethaline 1:2 is formed by mixing choline chloride and ethylene glycol in 1:2 molar proportion, propeline 1:3 is formed by mixing choline chloride and propylene glycol, with less nocive character than ethylene glycol, in 1:3 molar proportion.
More precisely, this data set contains for the two DES :
- Bulk properties of the pure DES : density, viscosity and conductivity depending on temperature and DES water content;
- Electrochemical windows of the pure DES on Pt and Ag working electrodes;
- Electrochemical silver leaching results : linear sweep voltamograms, chronoamperograms, chronopotentiograms, faradaic yields;
- Electrochemical silver kinetics parameters : diffusion coefficients, alpha and k° charge transfer parameters, and related raw cyclic/linear voltammograms and chronoamperograms
- Electrochemical deposition of silver : SEM-EDX and XRD raw data English (2024-04-08).
